Teije Middelkoop Group
Institute of Molecular Genetics
Czech Academy of Sciences

The shape of an animal arises in a species-specific, step-wise fashion during embryonic development. During this sequence of events, collectively referred to as ‘embryo morphogenesis’, the embryo constantly remodels its shape. We are interested in the force-generating mechanisms that drive these shape changes.
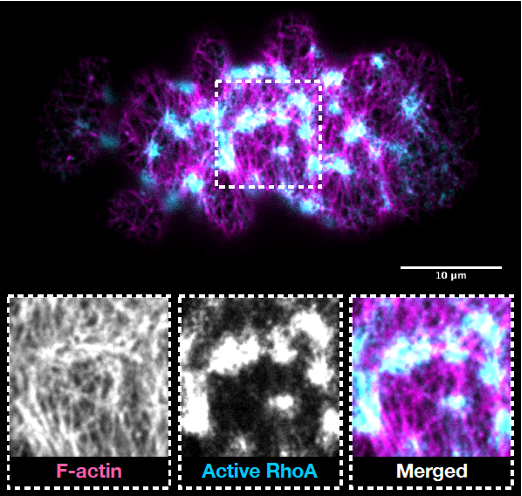
The forces that drive embryo morphogenesis are generated by the cytoskeleton of embryonic cells and mainly arise in the actomyosin cortex: a dense two-dimensional network of cross-linked actin polymers residing directly underneath the plasma membrane. By pulling and twisting actin polymers, myosin motors and numerous accessory proteins, can generate active forces and torques within the cortical layer. Tight spatiotemporal regulation of these molecular-scale forces ultimately results in cellular-scale shape changes required for embryo morphogenesis.
We use Caenorhabditis elegans and related nematode species to study how molecular forces generated within the actomyosin layer give rise to morphogenetic shape changes in early embryos. To this end, we combine the strength of C. elegans genetics with time-lapse imaging of early embryos (both at high-resolution and at super-resolution), quantitative image analysis and biophysical modelling.
Future projects
In general, we aim to understand how molecular-scale forces drive the cellular scale rearrangements required for morphogenesis. More specifically, we previously showed that active torque generation in the actomyosin cortex is driven by an actin polymerase of the Formin family. These active torques lead to rotatory movements of embryonic cells and organismal left-right symmetry breaking. In the future, we aim at understanding how Formin-dependent active torques, generated at the molecular level, are converted to rotatory behaviour at the cellular and organismal level.
Institute of Molecular Genetics
Czech Academy of Sciences
Videnska 1083
14000 Prague 4
Czech Republic
Since 2022
Group leader
Institute of Molecular Genetics, Czech Academy of Sciences
Prague, Czech Republic
2015-2022
Postdoc
Biotec, TU Dresden & Max Planck Institute of Molecular Cell Biology and Genetics
Dresden, Germany
2009-2014
PhD in developmental genetics
Hubrecht Institute
Utrecht, The Netherlands
Middelkoop TC, Garcia-Baucells J, Quintero-Cadena P, Pimpale L, Yazdi S, Sternberg P, Gross P, Grill SW. CYK-1/Formin activation in cortical RhoA signaling centers promotes organismal left-right symmetry breaking. PNAS, 118, 20, e2021814118, (2021)
Pimpale LG, Middelkoop TC, Mietke A, Grill SW. Cell lineage-dependent chiral actomyosin flows drive cellular rearrangements in early Caenorhabditis elegans development. Elife 9, e54930, (2020)
Naganathan SR*, Middelkoop TC*, Fürthauer S*, Grill SW. Actomyosin-driven left-right asymmetry: from molecular torques to chiral self organization. Curr Opin Cell Biol 38, pp. 24-30, (2016)